FIX-KD-19
PEDIATRIC NEOPRENE HIP BRACE WITH ROM ADJUSTMENT
Our hip brace FIX-KD-19 is perfect for supporting and off loading the hip joint.
Special features
 | HIP BRACE |
 | 1st CLASS MEDICAL PRODUCT |
 | 24/7 THERAPY |
 | WATERPROOF |
 | BREATHABLE |
 | MAGNETIC JOINT 1R ORTHODESIGN |
 | CAST REPLACEMENT |
Transient synovitis
It is one of the most common causes of joints infections. It is called also irritable hip. Transient synovitis usually affects children between three and ten years old and boys are affected two to four times as often as girls. The condition causes the inflammation of many joins: knees, ankles, wrists,elbows and hips. The reason is unknown. Together with inflammation occur fever or rash. It is important to do a lot of tests to differ the transient synovitis and viruses or bacteria.
Transient synovitis is painful and may reduce joint mobility. Due to this fact the joint support is very important. Our hip brace FIX-KD-19 is perfect for supporting and off loading the hip joint.
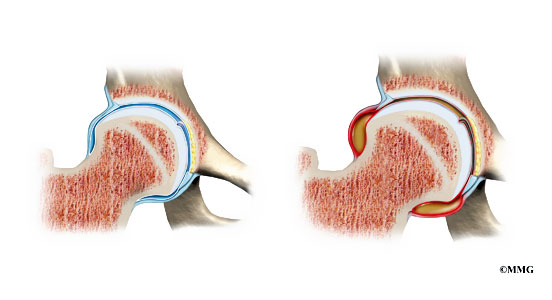
Transient synovitis
Indications
- Transient synovitis
- Perthes Disease (avascular necrosis)
- Hip deformations
- Hip dislocation
- Femoral fractures
- Acetabular labrum injury
Product’s description
Hip brace FIX-KD-19 is made of thin, colorful neoprene which provides constant compression and warmth of the hip girdle. The brace is equipped with aluminum splint and range of motion adjustment in every 15 degrees. The splint supports and offloads the overused joint and prevents against the malalignment. Also, it reduces the excessive hip rotation, abduction and adduction. Smooth adjustment of range of motion provides excellent joint control or immobilization.
The 1R Orthodesign orthopedic brace is a novel approach to the topic of adjustable-angle orthopedic braces. The innovativeness of the brace lies in the fact that we have applied an angle adjustment of 15 degrees in a clock with a diameter not exceeding 30 mm. An additional advantage of the brace is that the adjustment is not done using Allen screws, as it was in the previous generation of the brace, but using inserted steel rivets, which are easily removed from the angle sockets using a magnet included with the brace. The icing on the cake is the locking ring, which, thanks to the ratchet system, will prevent the previously set angle from being automatically readjusted. In knee braces, the braces have a shin offset, which allows for more precise fitting of the knee brace, and therefore more accurately realizes the regulation of joint mobility. In other products, the braces have a straight shape. An oval element is mounted on the underside of the clock to which a gripper is glued, which is essential for attaching soft 3D side cushions to the clock. These braces, using an orthopedic key offered by Reh4Mat, can be individually adjusted to the shape of the patient's limb in some cases by slightly bending them. Download the 1R orthodesign brace adjustment instructionsPediatric hip brace FIX-KD-19 is made of thin lightweight ActivePren™ and UniPren™. Hip compression and stabilization reduces the pain and inflammation. What is more, the neoprene is waterproof and the child may take a shower wearing our brace.
Day-to-day using of our hip brace FIX-KD-19 supports overused hip joint and reduces the inflammation due to injury or chronic condition. The brace is much effective and lighter than ordinary plaster cast.
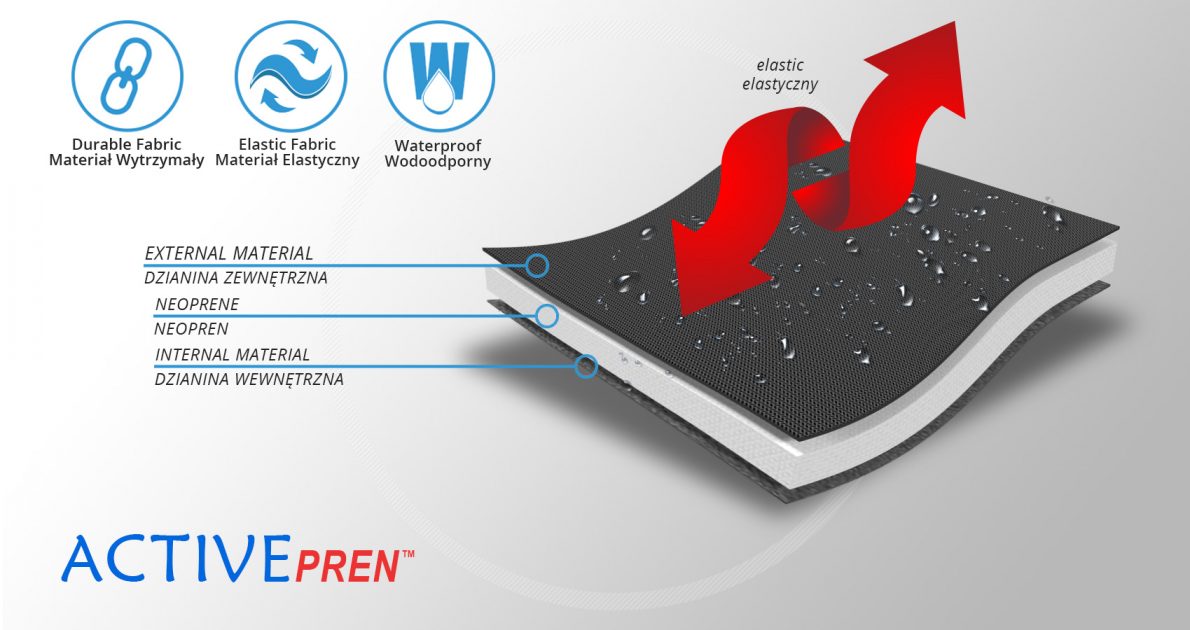
ActivePren™ is an active three-layer material consisting of two elastic jersey cover fabrics and a core made of neoprene foam. This material is characterized by softness and high flexibility. A very important advantage of this material is the fact that it is not a knitted product, it does not have thick fibers, so that the weaves of the material do not imprint on the patient's skin and do not cause abrasionsin places of high compression. Products made of ActivePren are the strongest and most effective stabilizing orthoses available on the market.
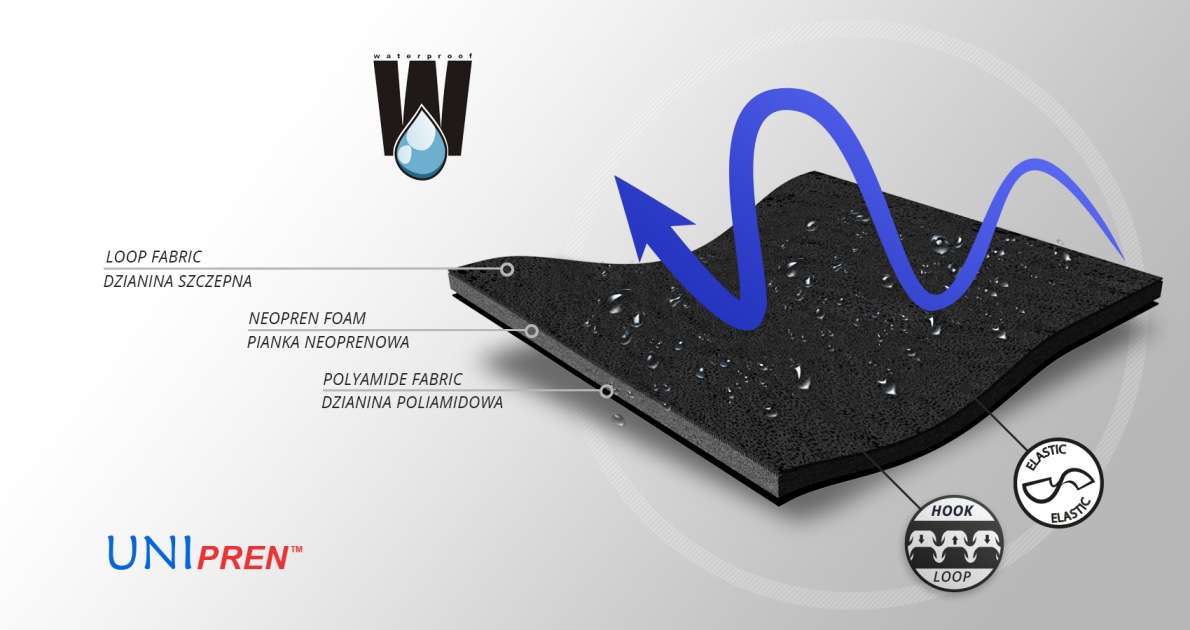
UniPren™ is a universal 3-layer material consisting of an external elastic polyamide cover knit with a self-adhesive function, an internal neoprene foam core and an elastic jersey cover knit. This material is characterized by softness and very high flexibility. A very important advantage of this material is the fact that it is not a knitted product, it does not have thick fibers, so that the weaves of the material do not imprint on the patient's skin and do not cause abrasionsin places of high compression. Products made of UniPren™ are the strongest and most effective stabilizing orthoses available on the market. Self-adhesive function, the raw material makes it much easier to use.
Available sizes
| Size | Waist circumference (A) |
Thigh circumference (B) |
Typical age | How to measure |
|---|---|---|---|---|
| 1 | min 40 – max 60 cm | min 25 – max 35 cm | 2 – 9 years | 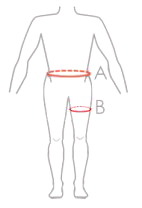 |
| 2 | min 45 – max 70 cm | min 30 – max 40 cm | 8 – 12 years |
Right and left hip specific .
Total length of the product:
1: 28 cm
2: 37 cm
The product is sent in a random color.

Downloads
Accessories to be used with this product:
ON OUR WEBSITE WE PRESENT MEDICAL DEVICES.
USE THEM ACCORDING TO THE INSTRUCTIONS FOR USE OR LABEL.
MANUFACTURER / ADVERTISING ENTITY: REH4MAT Sp. z o.o.





 Class I medical device in accordance with the Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017 on medical devices.
Class I medical device in accordance with the Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017 on medical devices.